inno.N's Production Site
With a global Standard IT system and priority placed on the data integrity, inno.N operates a quality management system in accordance with the standards of cGMP/EU GMP.
Enterprise Quality Management System
-
Quality
ManagementPolicies to improve product quality are established and a quality management system is operated. -
Quality
AssuranceThe quality of drugs is guaranteed by meeting cGMP standards. -
Quality
ImprovementThrough technological support, inno.N aims not only to offer good products, but those that meet some of the most stringent quality standards. -
Quality
AuditsThrough continuous quality audits, one of the most thorough quality management systems is maintained and managed.
About the quality system

Quality Policy
OUR MISSION
Heal the World for a Better Life
Code of Conduct
- We always listen to our customers and do our best to respond to their needs, fulfilling our social responsibilities for the patients suffering from disease and their families.
- We constantly improve with the New Frontier spirit for better quality.
- We follow principles and standards for quality and act honestly.
- We strive to have the appropriate members and systems to improve quality.

Data Integrity
HK inno.N has established governance to ensure the quality and integrity of data related to pharmaceutical manufacturing, and actively promotes dissemination of the quality-first culture that prioritizes data integrity.
First, HK inno.N declares the policies and the Code of Ethics that reflect the philosophy of the Management on quality, and takes the lead in preventing data integrity issues by building systematic training programs and systems along with adequate allocation of resources.
Second, to form an open organizational culture, the Company promotes open communication and reporting on data integrity failures, errors of individuals/systems, or potential data integrity issues.
Third, systematic education and training are implemented to raise employees' awareness of data integrity, such as the importance of data integrity and its impact on quality.
Fourth, procedures and systems are established and in operation, enabling systematic management of data integrity, such as data risk assessment and management, data review, and periodic monitoring.
In particular, HK inno.N records/manages all GMP-related activities in accordance with the most up-to-date global GMP regulations to enhance the reliability of GMP data for pharmaceutical products of the Company.
Internally, the Company distributes DI Letters to employees for embedding the quality-first mindset, implements data integrity training and evaluations on a regular basis, and continues to promote various activities to raise awareness of data integrity.
- MES, Manufacturing Execution System
- LIMS, Laboratory Information Management System
- QMS, Quality Management System
- EDMS, Electronic Document Management System
| Frequency | Year 2023 | Year 2022 | Year 2021 | ||
|---|---|---|---|---|---|
| Education | Publication of DI Letter | Every month | 10 times * | 12 times | 12 times |
| DI training | Bi-annually | 2 times, all employees at manufacturing department (631 employees) |
2 times, all employees at manufacturing department (587 employees) |
2 times, all employees at manufacturing department (546 employees) |
|
| Committee | DI Committee | Quarterly | 4 times | 5 times | 3 times |
| Working-level Committee on DI | Quarterly | 4 times | 5 times | 3 times | |
| Audit | Self-audit on DI | Three times a year | 3 times | 3 times | 3 times |
* Published from February to November since year 2023
One of the most cutting-edge production facilities for pharmaceutical products in Korea that meets global standards, the Osong Plant is where high quality oral solid dosage products, Oncology Injectables and IV fluids are manufactured.

-
01
Compliance with cGMP/EU GMP
-
02
Cutting-edge manufacturing facilities and an IT system for the full production process
-
03
Production technologies and hands-on experience from over 30 years in the industry
-
04
Compliance with global standards for safety and environment
| Address | 239, Osong Saengmyeong 2 Ro, Osong-Eup, Heungdeok-Gu, Cheongju-Si, Chungcheongbuk-Do, Korea |
|---|---|
| Area of the site | 146,013 m² |
| Area of the buildings | 58,896 m² |
| Key facilities | 12 buildings specializing each in the manufacturing of oral solid dosage products, Oncology Injectables and IV fluids |
| Dosage forms of products | Oral solid dosage products, Oncology Injectables and IV fluids |
| Certifications | KOSHA-MS, ISO 45001, ISO 14001, KGMP |
Oral solid dosage products


Oncology Injectables

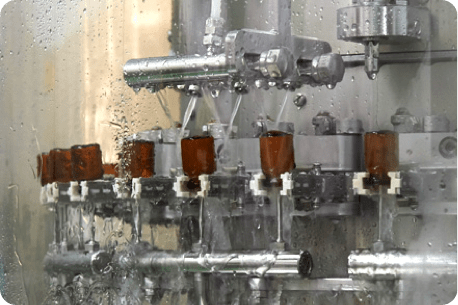
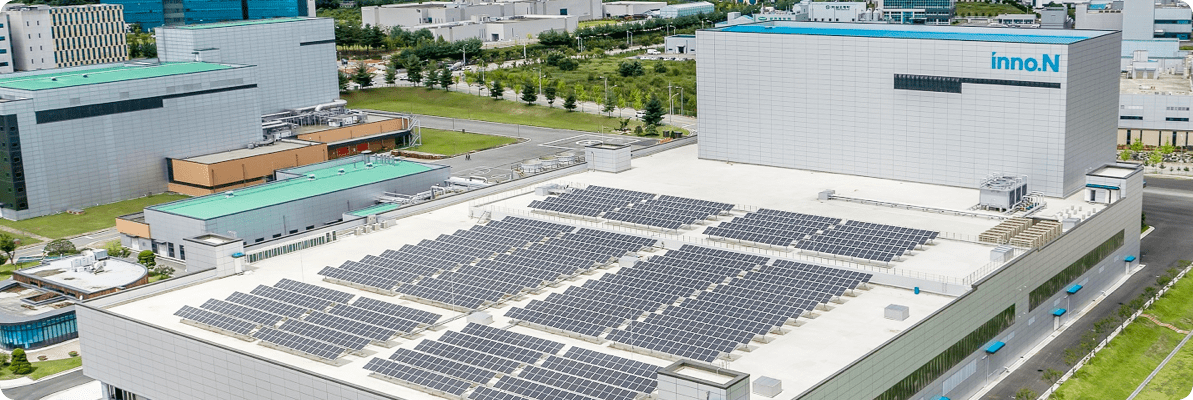
One of the largest smart factory in Korea with the latest automated facilities, the Osong IV Plant manufactures some of the highest quality IV products.
-
01
One of the largest production capacities in Korea with the latest automated facilities
-
02
A smart factory that operates based on Big Data analysis and forecasting
-
03
An environment-friendly production facility using renewable energy sources such as solar power
-
04
A global quality management system is implemented to ensure the highest quality in products
| Address | 239, Osong Saengmyeong 2 Ro, Osong-Eup, Heungdeok-Gu, Cheongju-Si, Chungcheongbuk-Do, Korea |
|---|---|
| Area of the site | 146,013 m² |
| Area of the buildings | 32,191 m² |
| Key facilities | Production lines in the IV building, automated warehouses and utilities |
| Dosage forms of products | Oral solid dosage products, Oncology Injectables and IV fluids |
| Certifications | KOSHA-MS, ISO 45001, ISO 14001, KGMP |
IV fluids






IV fluids, cephalosporin antibiotics (finished products, APIs), general APIs are manufactured and supplied to domestic and international customers.

-
01
A CMO of over 20 years with a major Japanese pharmaceutical firm
-
02
Specialized technologies for IV fluid production and automated facilities that meet GMP standards
-
03
Production facilities entirely dedicated to the API of K-CAB tablets, Tegoprazan
-
04
Over 20 years’ experience in manufacturing antibiotics of aseptic processing
| Address | 20 Daeso Sandan-Ro, Daeso-Myeon, Eumseong-Gun, Chungcheongbuk-Do, Korea |
|---|---|
| Area of the site | 58,278m² |
| Area of the buildings | 28,411m² |
| Key facilities | Twenty-five buildings including those dedicated for IV fluids, Tegoprazan, cephalosporin antibiotics, and TPN. |
| Dosage forms of products | IV fluids, oral solid dosage products, API for cephalosporin, General API |
| Certifications | KOSHA-MS, ISO45001, KGMP, PMDA, Iran FDA |
Tegoprazan


IV fluids


Antibiotics
(Cephalosporin)


Antibiotics
(Cephalosporin API)


With over 30 years of experience in manufacturing biopharmaceutical products, the Plant supplies its products to domestic and international customers.

-
01
Vaccine production facilities that are GMP-compliant
-
02
With over 20 products registered in a number of countries including Thailand
-
03
A quality management system to ensure the manufacturing of premium quality products
-
04
Production technologies and hands-on experience from over 30 years in the industry
| Address | 811 Deokpyeong-Ro, Majang-Myeon, Icheon-Si, Gyeonggi-Do, Korea |
|---|---|
| Area of the site | 34,751 m² |
| Area of the buildings | 14,492 m² |
| Key facilities | 10 buildings including those specialized for the production of vaccines and Epokine, and for quality management |
| Dosage forms of products | Biological products (e.g. vaccines and genetic recombination drugs), antibiotic injections |
| Certifications | KOSHA-MS, KGMP, TFDA, NADFC, Philippines FDA |
Injections


Epokine
Vaccines


With the establishment of a highly reliable GMP Cell Center, HK inno.N has been leading the industry in the development and manufacturing of Advanced Therapy Medicinal Products.

-
01
One of Korea’s largest automated production facilities with a closed system
-
02
Cell & Gene Therapy production facilities that are GMP-compliant
-
03
A top-notch quality management system to ensure high quality products are manufactured
-
04
Infrastructure for the development and optimization of CGT processes
| Address | Building D, 947 Hanamdaero, Hanam-Si, Gyeonggi-Do, Korea |
|---|---|
| Area of the site | Approximately 1,508 m2 or 457 pyeong |
| Key facilities | Process development lab, Quality testing and analysis lab, Aseptic manufacturing room, Storage room |
| Product formats manufactured | Advanced Therapy Medicinal Products (for Cell & Gene Therapy) |
| Permission | Permission for Manufacturing Advanced Biological Products (2022.08) Permission for Management Business of Human Cells (2023.09) Permission for Cell Processing Facility (2024. 12) |
Process development lab


Quality testing and analysis lab


Aseptic manufacturing room