Achievements
Based on the differentiated research capabilities and experience in developing a wide range of products,
inno.N continues its R&D for next-generation pharmaceutical products.
R&D results
Based on the differentiated research capabilities and experience in developing a wide range of products, inno.N continues its R&D for next-generation pharmaceutical products.

- Launched as a treatment for GERD in 2019
- Addressed the shortcomings of PPI and H2RA treatments.
- Excellent efficacy compared to competing P-CAB products.
- Over 100 billion won worth of products have been prescribed within the shortest span in time of all novel drugs in Korea.
-
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
-
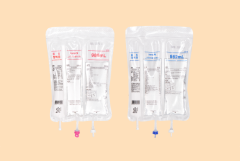
OMAPPLUSONE Inj. and OMAPPLUSONE PERI Inj., Total Parenteral Nutritionare(TPN), are launched
-
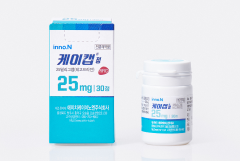
K-CAB 25mg is launched in Korea
K-CAB selects excellent technology in the field of life science for '2023 Industrial Technology Performance'(National Academy of Engineering of Korea)y
K-CAB won the '2023 Industrial Research(IR) 52 Jang Yeong-sil Award' -

K-CAB released as a ODT(Orally Disintegrated Tablet) form
K-CAB launches in China ('Taixinzan')
K-CAB enters phase 3 clinical trials in US -
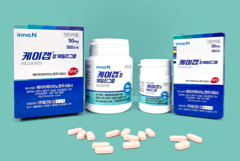
Technologies applied to manufacturing K-CAB are exported to U.S.A. and Canada
-
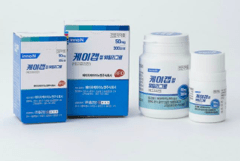
“K-CAB” received USFDA approval for Phase 1 clinical trials.
-
K-CAB, the 30th novel drug to be developed and registered in Korea, is launched in tablet form.
-

K-CAB (with Tegoprazan as the active ingredient) receive approval from the Korean Ministry of Food & Drug Safety to be used as a drug for GERD.
Korea’s 30th novel drug -

Technologies for CJ-40001 (second generation EPO) are exported to the China-based company, NCPC Genetech Biotechnology.
-

Technologies for CJ-40001 (second generation EPO) are exported to the Japan-based company, YL Biologics.
-
- 2016
- 2014
- 2013
- 2012
-
Machkhan, an improved and modified drug for hypertension, is launched
A drug with the combined active ingredients of ARB(angiotensin II receptor blocker) and CCB(calcium-channel blocker)
The first drug to combine the active ingredients of Candesartan /Amlodipine -

K-CAB, a novel drug developed in-house to treat GERD is approved by the Korean Ministry of Food and Drug Safety
A novel drug in the category of P-CAB drugs (potassium competitive acid blockers).
Related technologies are exported to Luoxin, China (2015)
Application is filed for approval as a novel drug (2017) -

Vogmet, an improved and modified drug to treat diabetes, is launched.
A drug with the combined active ingredients of α-glucosidase and biguanides
The first drug to combine the active ingredients of voglibose and metformin. -

Tegoprazan,
Selected by the government as a key initiative for the development of novel drugs in all phases -

Exone, a combined drug treatment for hypertension, is launched.
A drug with the combined active ingredients of ARB(angiotensin II receptor blocker) and CCB(calcium-channel blocker)
A drug with the combined active ingredients of Valsartan and
Outstanding photo-stability due to differentiated technologies -

inno.N is selected by the Ministry of Health and Welfare as an ‘innovative pharmaceutical firm’.
inno.N is recognized for its outstanding R&D for novel drugs and export capabilities.
-
- 1998
- 1995
- 1992
- 1986
-
Epokine, a treatment for anemia, is launched.
inno.N becomes the first company in Korea and the third company in the world to develop an EPO drug. -

Citopcin injection acquires certification for ‘domestic new technologies’ and is given the Chang Young Sil Award.
The first drug in Korea to use TOF, a biological substance -

Anti-hangover beverage Condition is launched.
Condition becomes the first anti-hangover beverage to be introduced in the Korean market -
Hepaccine-B, a vaccine for Hepatitis B, is launched.
Hepaccine-B is the first vaccine for Hepatitis B to be developed in Korea and the second to be developed in the world.
Certification from the WHO (1993)