K-CAB, HK inno.N’s new GERD treatment received recommendation for marketing authorization in India
December 2, 2024
K-CAB, HK inno.N’s new GERD treatment received recommendation for marketing authorization in India
K-CAB Tablet 25mg & 50mg, close to obtaining MA from India, the world’s most populous country
Export contract between HK inno.N and Dr. Reddy’s Laboratories, an India-based pharmaceutical company, already in place from 2022
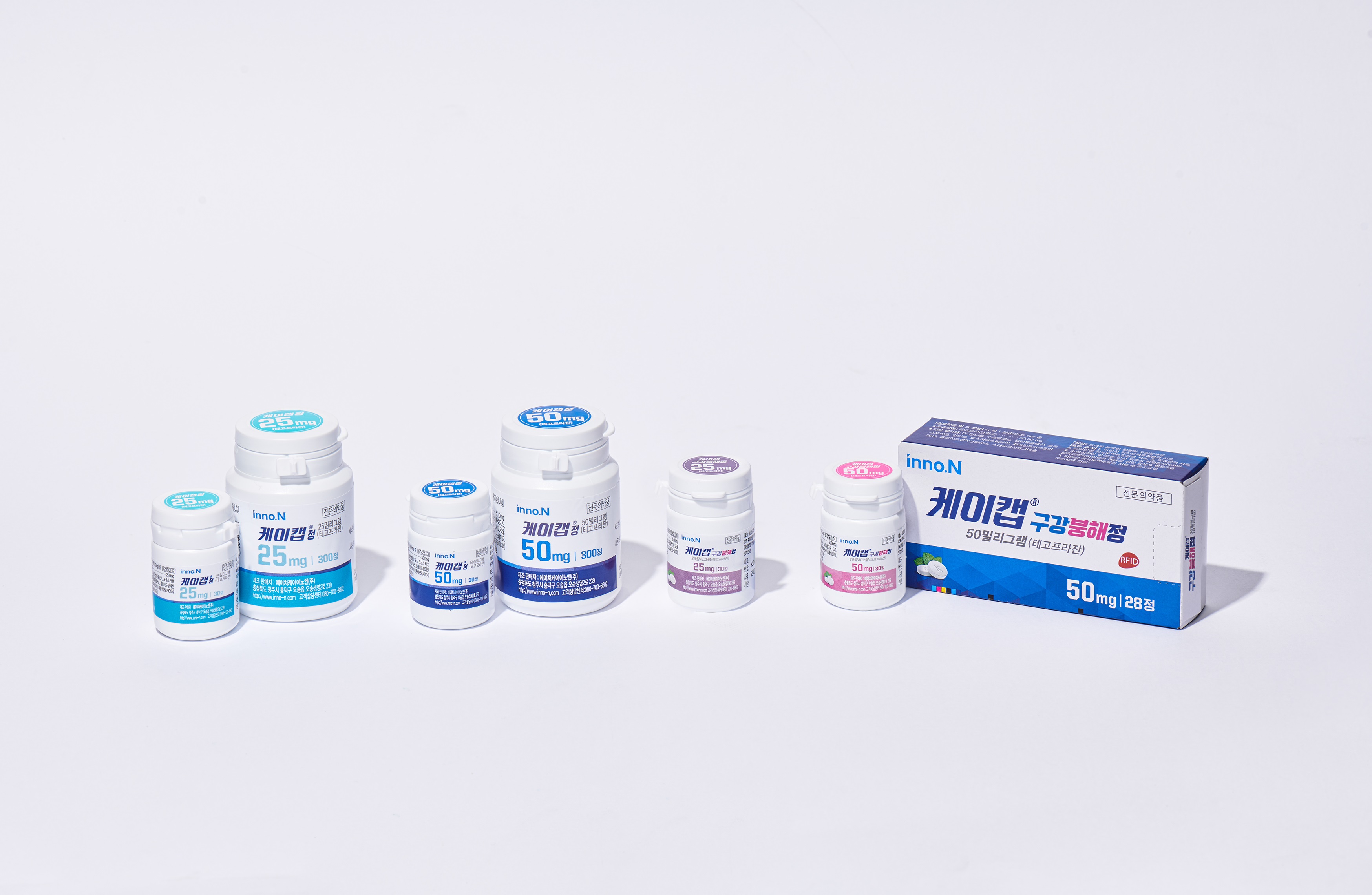
HK inno.N announced on Monday (December 2nd) that it received a recommendation for granting marketing authorization of its gastroesophageal reflux disease (GERD) treatment, K-CAB Tablet, from the Subject Expert Committee (SEC) under the Central Drugs Standard Control Organization (CDSCO) of India.
The recommendation applies to K-CAB Tablet 25 mg and 50 mg. The SEC recommended approval for the 50 mg tablet under indications of erosive GERD, non-erosive GERD, and gastric ulcers. For the 25 mg tablet, the recommendation is for △maintenance therapy after treatment of erosive GERD.
For antibiotic combination therapy for eradication of Helicobacter pylori, the SEC recommended conducting a local phase 3 clinical trial for K-CAB, citing limited global data available in relation to the eradication of Helicobacter pylori using K-CAB Tablet and varying patterns of antimicrobial-resistant H. pylori across different countries.
In 2022, HK inno.N signed an export contract with Dr. Reddy’s Laboratories, a pharmaceutical company in India, for distribution of the products across seven countries, including India, South Africa, and Eastern Europe.
India, with the largest population in the world at 1.45 billion, is the fourth-largest market for peptic ulcer medications, following China, the U.S., and Japan.
An official of HK inno.N said, “we expect that K-CAB will obtain marketing authorization in India next year following the recommendation for granting MA this time,” and “K-CAB’s marketing presence will grow even stronger with the drug obtaining more MA and the subsequent product release across major countries in the global market.”
K-CAB, the 30th new drug in South Korea, is a novel potassium competitive acid blocker (P-CAB) for GERD treatment. It is characterized by the rapid onset of action within one hour after administration and proven efficacy and safety even with long-term use for up to 6 months.
Following its first launch in South Korea, K-CAB succeeded in the market entry to a total of 46 countries worldwide, including China, US, India and Latin America. For each country of market entry, approval from the relevant authority and product release are in progress, and K-CAB has been officially released in 15 countries to date. K-CAB recorded a total outpatient prescription sales of KRW 160.2 billion from January to October for this year alone in South Korea. The product has been the steady No. 1 market leader in peptic ulcer medications for four consecutive years since its launch. (The End)
[Reference information]
■ P-CAB: Potassium Competitive Acid Blocker
■ CDSCO: Central Drugs Standard Control Organization
■ SEC: Subject Expert Committee
■ Size of the global peptic ulcer medications market at a glance (※ Source: IQVIA, for 2021)
: Total $18 billion (approx. KRW 25 trillion)
- China: approx. $3.1 billion (approx. KRW 4.6 trillion, ranked 1st)
- United States: approx. $2.8 billion (approx. KRW 3.9 trillion, 2nd)
- Japan: approx. $2.2 billion (approx. KRW 3 trillion, 3rd)
- India: approx. $760 million (approximately KRW 1 trillion, 4th)
■ The export contract between HK inno.N and Dr. Reddy’s Laboratories takes effect in the following 7 countries:
▲India ▲Republic of South Africa ▲Russia ▲Kazakhstan ▲Uzbekistan ▲Ukraine ▲Belarus