HK inno.N’s K-CAB enters Australian and New Zealand markets
January 6, 2025
HK inno.N’s K-CAB, new drug for Gastroesophageal Reflux Disease (GERD) treatment, enters Australian and New Zealand markets
Signed a finished product export agreement for K-CAB with Southern XP, an Australian pharmaceutical company
K-CAB has now entered 48 countries worldwide, including the US, China and Latin America... aiming for market entry into 100 countries by 2028

K-CAB, HK inno.N’s new drug for gastroesophageal reflux disease (GERD), is expanding its presence into Australian and New Zealand markets.
HK inno.N announced on Monday (January 6th) that the company recently signed an agreement for exporting finished products of K-CAB tablets (active ingredient tegoprazan), its new GERD treatment, to Australia and New Zealand with Southern XP, an Australian pharmaceutical company.
Under this agreement, Southern XP will hold exclusive rights to obtain regulatory approval and for commercialization of K-CAB in Australia and New Zealand. The agreement is applicable to the two products: ▲K-CAB tablet 50 mg and ▲K-CAB tablet 25 mg.
Southern XP is based in Australia with over 20 years of experience in the pharmaceutical industry, specializing in obtaining the regulatory approvals for and distribution of pharmaceuticals in Australia and New Zealand.
The pharmaceutical markets in the two countries are valued at approximately KRW 22 trillion in total as of 2023, of which the market for peptic ulcer medications is valued at about KRW 150 billion.
HK inno.N’s CEO Dalwon Kwak commented, “The value of K-CAB as a new drug of South Korea is recognized across different continents worldwide, showing a steady increase in the product’s influence in the global market of peptic ulcer medications” and added, “With the target of market entry into 100 countries worldwide by 2028, K-CAB’s global competitiveness will continue to grow.”
K-CAB, the 30th new drug of South Korea, is a P-CAB class new drug for GERD treatment. It is characterized by rapid onset of action within one hour after administration and proven efficacy and safety even with long-term use for up to six months.
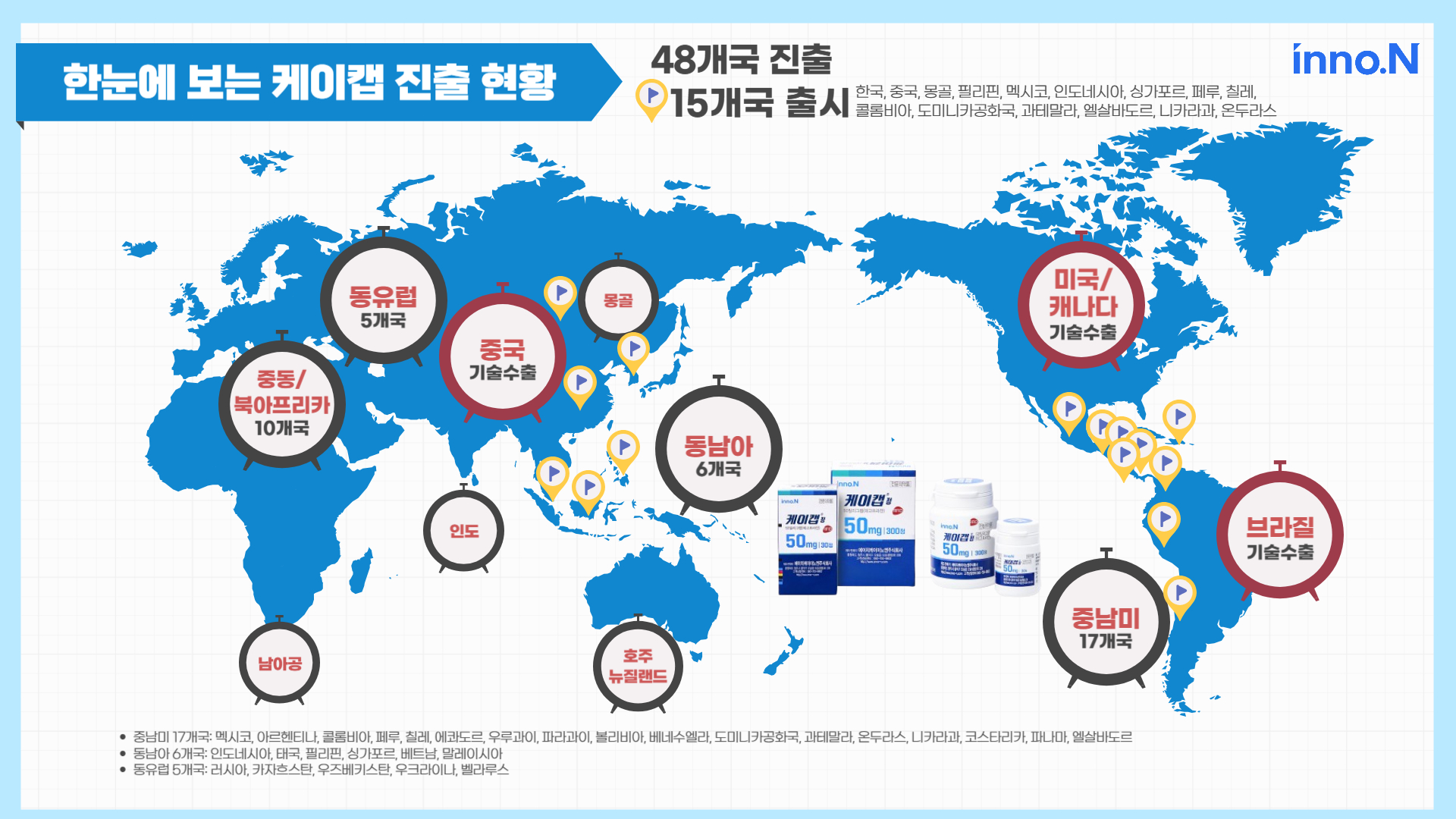
Following its first launch in South Korea, K-CAB succeeded in the market entry to a total of 48 countries worldwide, including the US and China. The product has been officially released in 15 countries to date, demonstrating its growing global market presence. K-CAB recorded a total outpatient prescription sales of KRW 177.7 billion from January to November of 2024 in South Korea. The product has been the steady No. 1 market leader in peptic ulcer medications for five consecutive years. [The End]
[Reference information]
■ P-CAB: Potassium Competitive Acid Blocker
■ Source on the size of the Australian/New Zealand pharmaceutical market and peptic ulcer medications market: IQVIA data, 2023
■ Overview of K-CAB’s market presence worldwide (48 countries in total):
- South Korea, China, Mongolia, India, South Africa, the United States, Canada, Australia, New Zealand
- 6 Southeast Asian countries: Indonesia, Thailand, Philippines, Vietnam, Singapore, Malaysia
- 5 Eastern European countries: Russia, Kazakhstan, Uzbekistan, Ukraine, Belarus
- 18 Central and South American countries: Brazil, Mexico, Argentina, Colombia, Peru, Chile, Ecuador, Uruguay, Paraguay, Bolivia, Venezuela, Dominican Republic, Guatemala, Honduras, Nicaragua, Costa Rica, Panama, El Salvador
- 10 Middle Eastern/North African countries
■ Countries with official product release of K-CAB (15 countries in total): South Korea, China, Philippines, Mongolia, Mexico, Indonesia, Singapore, Peru, Chile, Colombia, Dominican Republic, Guatemala, El Salvador, Nicaragua, Honduras