K-CAB, HK inno.N’s new GERD treatment approved in Malaysia
September 26, 2024
K-CAB, HK inno.N’s new GERD treatment approved in Malaysia
K-CAB obtains marketing authorization in Malaysia following the recent of news of MA in 6 Latin American countries...Ongoing achievements of MA and product release of K-CAB in the global market
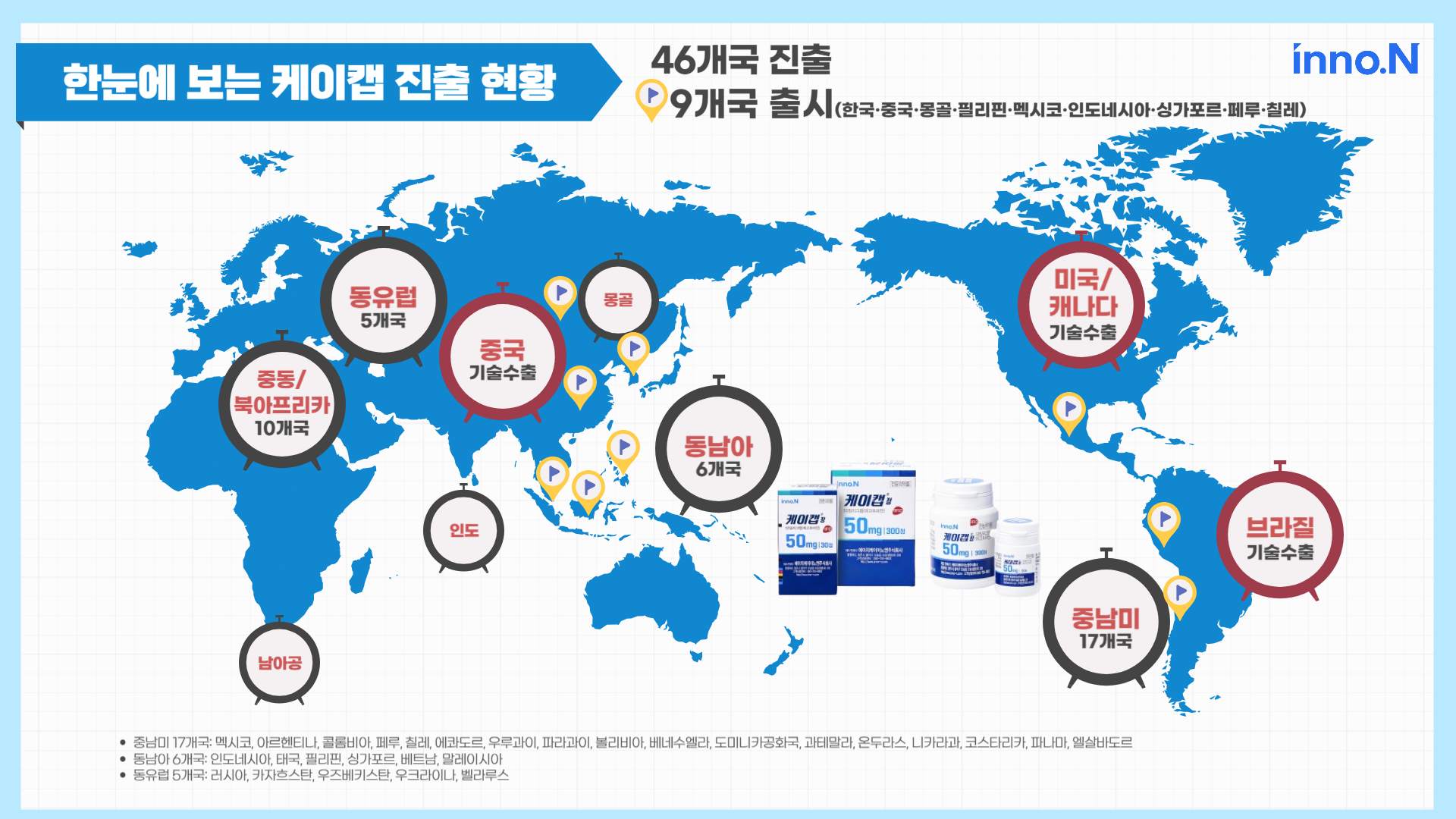
K-CAB succeeded in the global market entry across 46 countries including South Korea, and product launch in 9 countries...Expansion of K-CAB’s global market presence continues
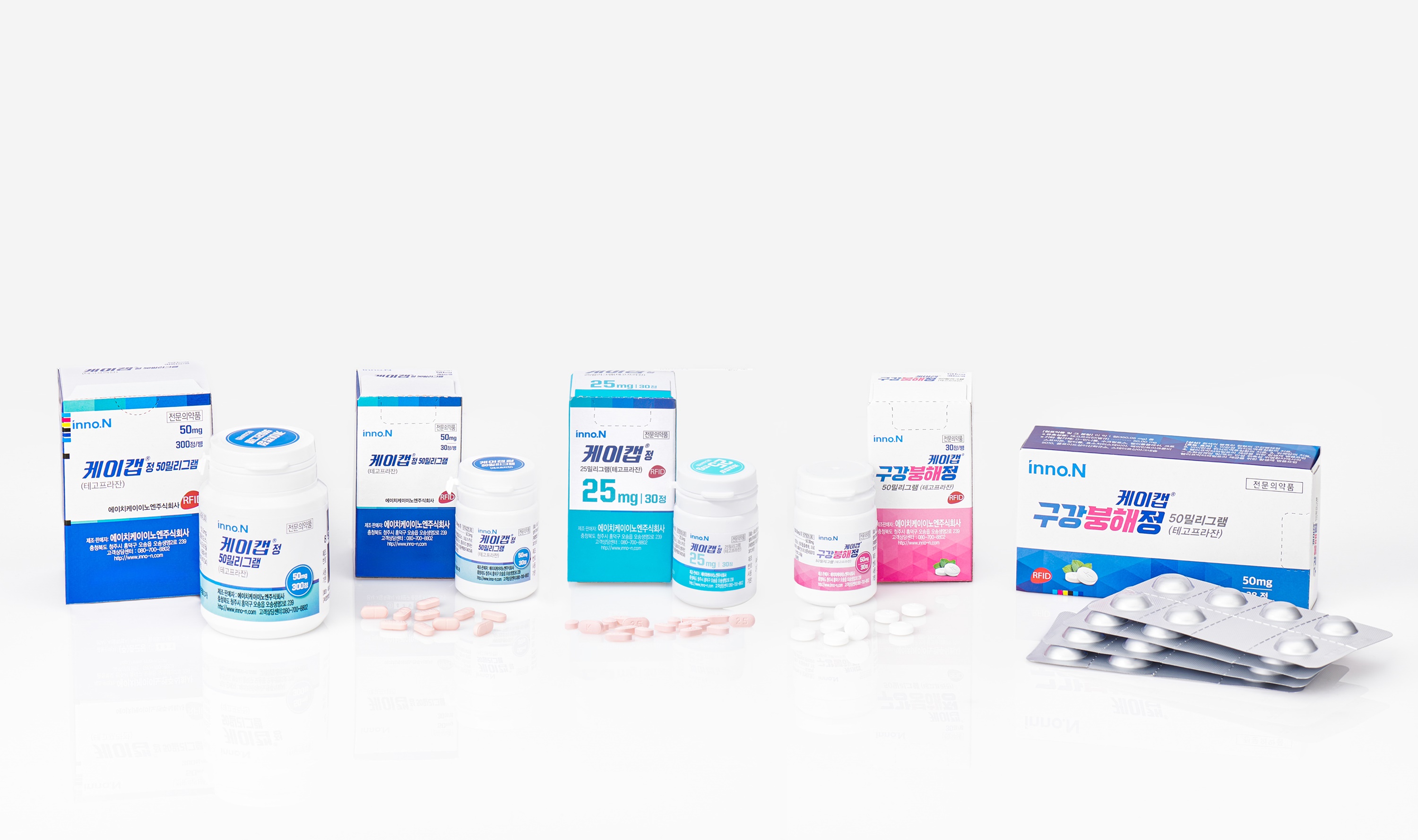
K-CAB, HK inno.N's novel drug for Gastroesophageal Reflux Disease (GERD) treatment, obtained marketing authorization in Malaysia.
HK inno.N announced on September 26 that K-CAB (tegoprazan) obtained marketing authorization (MA) from National Pharmaceutical Regulatory Agency (NPRA) of Malaysia. The MA follows an agreement signed in 2021 with No. 1 Malaysian pharmaceutical company, Pharmaniaga Logistic Sdn Bhd, for the export of K-CAB drug products.
K-CAB is approved for the four indications: △ treatment of Erosive Gastroesophageal Reflux Disease △ treatment of Non-Erosive Gastroesophageal Reflux Disease △ treatment of Gastric Ulcer △ Antibiotic combination therapy for eradication of Helicobacter pylori in patients with peptic ulcers and/or chronic atrophic gastritis. K-CAB will be marketed under the same local product name of ‘K-CAB,’ and preparation is underway for its product launch in Malaysia in the first half of 2025.
K-CAB’s entry to global pharmaceutical markets spans across 45 countries including the US and China, of which nine countries including South Korea had official product launches. In Southeast Asia, K-CAB entered the six largest economies in the region, which includes ▲Philippines ▲Indonesia ▲Singapore. The Southeast Asian market for peptic ulcer medications is estimated at approximately $520 million, with rising anticipation for its growth potential.
“K-CAB’s market entry to major countries in Southeast Asia will provide yet another momentum for its growing market presence,” commented Dalwon Kwak, CEO of HK inno.N. “Beyond Southeast Asia, we aim to establish K-CAB as a leading product of the P-CAB class drugs in the global market, raising the profile of drugs developed in South Korea.”
K-CAB, the 30th new drug in South Korea, is a P-CAB class new drug for GERD treatment. It is characterized by rapid onset of action within one hour after administration and proven efficacy and safety even with long-term use for up to six months. Since its domestic launch in 2019, K-CAB recorded a total outpatient prescription sales of KRW 761.1 billion to August 2024. The product has been the steady No. 1 market leader in peptic ulcer medications for four consecutive years since its launch. [The End]
[Reference information]
■ P-CAB: Potassium Competitive Acid Blocker.
■ K-CAB is launched in the following countries (9 countries/market entry to a total of 46 countries)
- South Korea, China, Mongolia, Philippines, Mexico, Indonesia, Singapore, Peru, Chile
■ K-CAB’s successful track record of market entry to Southeast Asia includes the following 6 countries: Indonesia, Thailand, Philippines, Singapore, Vietnam, Malaysia
■ Overview of K-CAB’s market presence in Southeast Asia
- Product release (3 countries): Philippines(Nov 2022), Indonesia(Jul 2023), Singapore(Aug 2023)
- Marketing authorization (2 countries): Malaysia, Thailand
- Marketing authorization under review: Vietnam
■ Estimation of the peptic ulcer drugs market size in Southeast Asia (for 2023): 526,800,000$ (Source: IQIVA ※ Based on the seven countries in the region: Indonesia, Vietnam, Thailand, Philippines, Singapore, Malaysia, Sri Lanka)